Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions
activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditionsSawaguchi, Takuma; Tsukada, Manabu; Yamaguchi, Tetsuji; Mukai, Masayuki
Clay Minerals, 51(2), p.267 - 278, 2016/05
Times Cited Count:7 Percentile:24.47(Chemistry, Physical)The dependences of the dissolution rate of compacted montmorillonite on activity of OH (a
(a -) and temperature (T) were investigated. The dissolution rate of montmorillonite (
-) and temperature (T) were investigated. The dissolution rate of montmorillonite (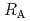 ) in compacted pure montmorillonite, which was formulized as
) in compacted pure montmorillonite, which was formulized as 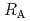 = 10
= 10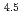 (a
(a -)
-)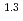 e
e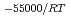 , was higher than that in the compacted sand-bentonite mixtures:
, was higher than that in the compacted sand-bentonite mixtures: 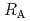 = 3500 (a
= 3500 (a -)
-)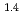 e
e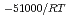 . The difference can be explained by considering the decrease in a
. The difference can be explained by considering the decrease in a - in the mixtures accompanied by dissolution of accessory minerals such as quartz and chalcedony. The dissolution rate model developed for pure montmorillonite is expected to be applied to bentonite mixtures if quantification of the decrease in a
- in the mixtures accompanied by dissolution of accessory minerals such as quartz and chalcedony. The dissolution rate model developed for pure montmorillonite is expected to be applied to bentonite mixtures if quantification of the decrease in a - is achieved somehow.
- is achieved somehow.
Ishidera, Takamitsu; Kurosawa, Seiichi*; Hayashi, Masanori*; Uchikoshi, Keiji*; Beppu, Hikari*
Clay Minerals, 51(2), p.161 - 172, 2016/05
Times Cited Count:4 Percentile:14.04(Chemistry, Physical)The sorption and diffusion behavior of Cs in illite-added compacted montmorillonite was investigated by through-diffusion experiment. The obtained distribution coefficient of Cs for the illite-added compacted montmorillonite was several times larger than that for the montmorillonite without illite, while no increase of effective diffusion coefficient was observed for the illite-added compacted montmorillonite. The dominant sorption site of Cs on illite is considered to be the frayed edge site (FES) considering the Cs concentration in this experiment. Therefore, the surface diffusion of Cs sorbing on the FES on illite surface was considered to be negligible in compacted montmorillonite.
Yamaguchi, Tetsuji; Sawaguchi, Takuma; Tsukada, Manabu; Hoshino, Seiichi*; Tanaka, Tadao
Clay Minerals, 51(2), p.279 - 287, 2016/02
Times Cited Count:7 Percentile:24.47(Chemistry, Physical)Alteration of bentonite-cement interfaces and accompanying changes in diffusivity of tritiated water was experimentally investigated using intact hardened cement specimens. The alteration by carbonate solution was accompanied by mineralogical changes at the interface and a decrease in the diffusivity to 70% of the initial value after 180-day period. Another alteration under silicate system contacting hardened cement and compacted bentonite was accompanied by mineralogical changes at the interface and a decrease in the diffusivity to 71% of the initial value after 600-day period. The changes in the diffusivity were much less than those observed for mixed specimens of granulated hardened cement and bentonite where the diffusivity decreased down to 20% of the initial value over 180 days. The results were extrapolated to 15 years under simple assumptions and showed good agreement with those observed in the cement-argillite interface at Tournemire URL. Such an explanation enhances our confidence in our assessment of alteration of cement-bentonite systems and can be a base for using our data and models in long term assessment of radioactive waste disposal.
Tachi, Yukio; Yotsuji, Kenji; Ito, Tsuyoshi; Suyama, Tadahiro
no journal, ,
The integrated sorption and diffusion (ISD) model was applied for systems coexisting multispecies Sr (divalent cation Sr and neutral SrSO
and neutral SrSO (aq)) in compacted montmorillonite. Effective diffusion coefficients (De) and distribution coefficients (Kd) of Sr in compacted Na-montmorillonite (dry density of 800 kg/m
(aq)) in compacted montmorillonite. Effective diffusion coefficients (De) and distribution coefficients (Kd) of Sr in compacted Na-montmorillonite (dry density of 800 kg/m ) saturated with three types of Na
) saturated with three types of Na SO
SO solutions (0.05, 0.1, 0.5 M) were measured by the trough-diffusion method. The De and Kd values decreased drastically with increasing porewater salinity. The De for multispecies Sr was determined as the harmonic weight-average considering the two species distribution and their log De values, based on comparison with reactive-transport calculations using the PHREEQC. As a result, the De trend could be quantitatively express by the ISD model considering multispecies contributions. The thermodynamic sorption model considering ion exchange reactions could provide reasonable account of Kd trend as functions of salinity.
solutions (0.05, 0.1, 0.5 M) were measured by the trough-diffusion method. The De and Kd values decreased drastically with increasing porewater salinity. The De for multispecies Sr was determined as the harmonic weight-average considering the two species distribution and their log De values, based on comparison with reactive-transport calculations using the PHREEQC. As a result, the De trend could be quantitatively express by the ISD model considering multispecies contributions. The thermodynamic sorption model considering ion exchange reactions could provide reasonable account of Kd trend as functions of salinity.
Okubo, Takahiro*; Ibaraki, Moe*; Tachi, Yukio; Iwadate, Yasuhiko*
no journal, ,
1H NMR relaxometry was applied for investigation of pore structure in compacted saturated montmorillonite with different salt concentration. The samples compacted to dry densities of 0.8 and 1.2 g/cm were saturated with pure water, 0.05, 0.10, 0.50, and 1.0 M NaCl solutions. Population of water in different hydrated layer corresponding to 1-, 2-, and 3 hydrated layer was estimated from longitudinal relaxation distributions. The population of water in 1-, 2-, and 3 hydrated layers changed as a function of NaCl concentration. The threshold to discriminate between inter- and intra-layer water is 3 hydrated layer, which is questionable from existence of 4 hydrated layer revealed by modelling of X-ray profile. The amount of water in 1- and intra-layer were negligible small for all conditions at dry density of 1.2 g/cm
were saturated with pure water, 0.05, 0.10, 0.50, and 1.0 M NaCl solutions. Population of water in different hydrated layer corresponding to 1-, 2-, and 3 hydrated layer was estimated from longitudinal relaxation distributions. The population of water in 1-, 2-, and 3 hydrated layers changed as a function of NaCl concentration. The threshold to discriminate between inter- and intra-layer water is 3 hydrated layer, which is questionable from existence of 4 hydrated layer revealed by modelling of X-ray profile. The amount of water in 1- and intra-layer were negligible small for all conditions at dry density of 1.2 g/cm . On the other hand, water in 2-hydrated layer was decreased instead of increment of water in 3-hydrated layer.
. On the other hand, water in 2-hydrated layer was decreased instead of increment of water in 3-hydrated layer.